Pipeline
‘1104
Potential Platform to Treat Allergic Diseases
We are developing ‘1104 for the treatment of patients with eosinophilic esophagitis (EoE) and other type 2 inflammatory allergic diseases. We have demonstrated the ability to deliver ‘1104 via subcutaneous (SC) and sublingual (SL) routes of administration in a preclinical model, with plans to advance both into the clinic. Sublingual dosing is unique in the allergic and autoimmune disease landscapes and could significantly impact the treatment landscape for patients.
Eosinophilic Esophagitis
EoE is a progressive allergic disease characterized by difficulty swallowing (dysphagia) and gastric reflux. It results from an elevated number of inflammatory immune cells, including but not limited to eosinophils, in the walls of the esophagus. Roughly 180,000 children and adults in the US have EoE1, and up to 80% of patients have secondary allergic conditions.2 Early disease control is critical to avoid thickening of the tissue of the esophagus, associated with disease progression.
Current treatment options include dietary management and medications, such as steroids and biologics, that target inflammatory pathways after the immune system has been overactivated. Steroid treatments require frequent dosing and are often associated with unwanted side effects, such as immune suppression. While some existing treatments reduce eosinophil levels and improve symptoms, they do not address the complex physiopathology of EoE and don’t work or may not be accessible for everyone, leaving many patients in need of other options.
A Phase 2a clinical trial evaluating ‘1104’s efficacy, safety and tolerability met its primary efficacy endpoint with a reduction in the peak eosinophil cell count in the walls of the esophagus.3 Additional analysis showed an improvement in patient-reported dysphagia symptoms compared to placebo. The data exhibited ‘1104’s broad mechanistic effect across a range of key immune cell types associated with EoE, a key differentiator from other therapies. ‘1104 led to an increase in regulatory B cells (Bregs) and T regulatory cells (Tregs), which are key immune cells associated with immune regulation. Additionally, ‘1104 caused a reduction in pro-inflammatory CD4+ and CD8+ T cells and a normalization of 15 key EoE genes considered to affect remodeling, eosinophil and mast cell count, inflammation, cell adhesion, and the epithelium lining. Safety assessment was favorable with no related serious adverse events or study drug discontinuations due to drug-related adverse events.
A larger Phase 2 trial investigating ‘1104 subcutaneous administration in EoE is planned for 2025.
Atopic Dermatitis
A proof-of-concept Phase 2 study in an additional Th2 allergic indication is planned to start in 2025.
Allergic Diseases
People with allergic diseases often experience symptoms even during treatment with currently available products. For more than 50 years, the number of people with allergic diseases has been rising and continues to climb.6
Recent clinical research across multiple allergic diseases has underscored the significance of targeting upstream pathways in the immune cascade, rather than focusing solely on single cytokines or immune cell types like eosinophils.
Given ‘1104’s immune homeostasis restoration mechanism, antigen-agnostic nature, unprecedented dosing optionality and clinical data observed in trials to date, we believe ‘1104 has the potential as a first line therapy across a number of allergic disease indications beyond EoE.
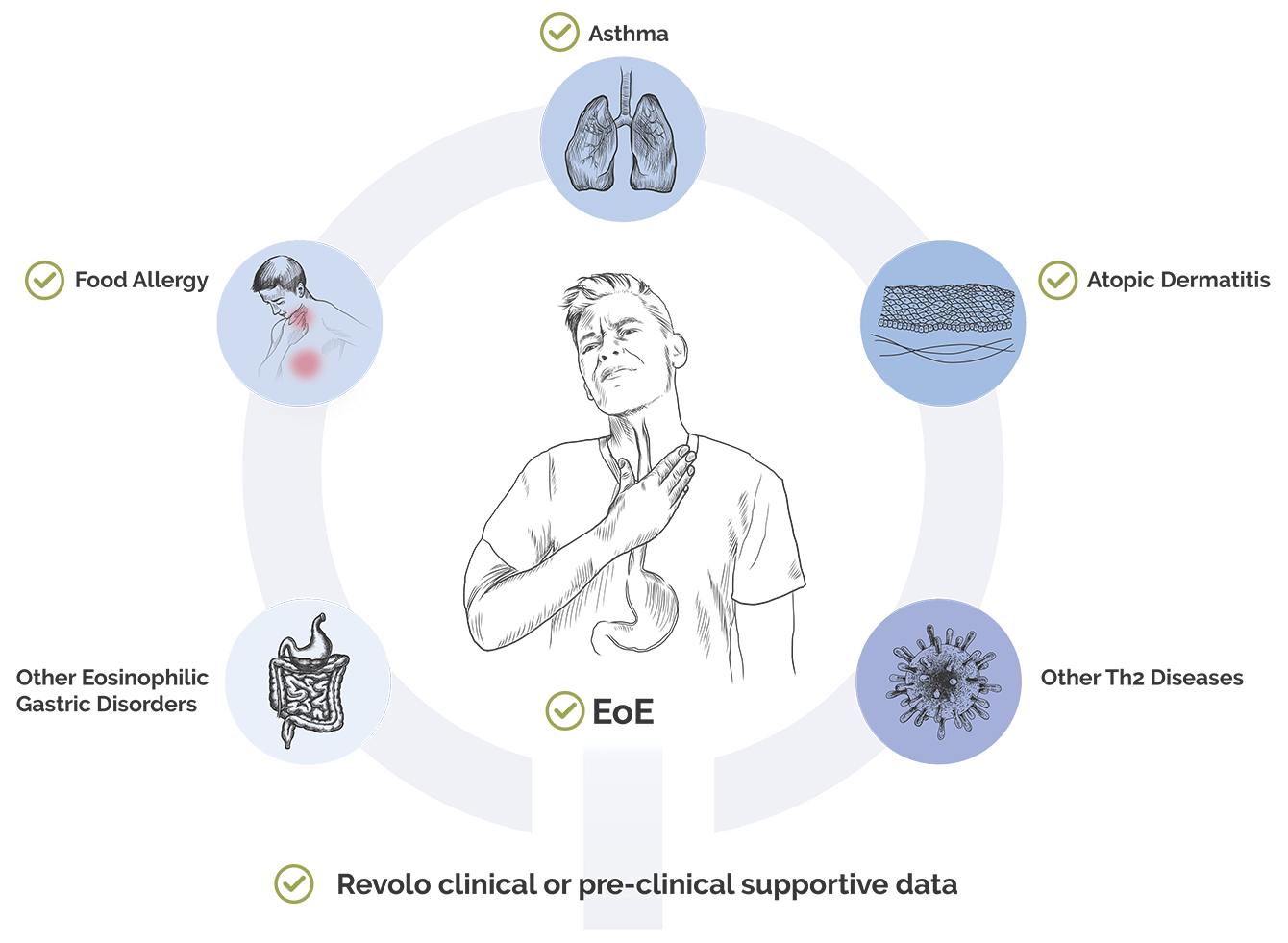
- Atopic Dermatitis: An estimated 10 million individuals live with moderate-to-severe atopic dermatitis in the U.S.,7,8 with many patients remaining untreated. Novel upstream mechanisms are crucial for addressing the needs of this patient population.
- Other Eosinophilic Gastric Disorders: There is a pressing need for innovative treatments for the large number of patients suffering from other eosinophilic gastric disorders beyond EoE, which account for up to 50,000 individuals, many of whom are thought to be undiagnosed.
- Asthma: There is a significant unmet need for the more than 7.5 million patients living with asthma in the U.S., refractory to steroids.9,10,11
- Food Allergy: More than 15 million people in the US are affected by one or more food allergies, highlighting the critical demand for new advancements and readily available treatments.12
- Other Th2 Diseases: Beyond asthma and atopic dermatitis, the development of novel therapies is essential for addressing the spectrum of Th2-driven diseases. This includes conditions such as chronic rhinitis with nasal polyps.
A proof-of-concept Phase 2 study in an additional Th2 allergic indication is planned to start in 2025.
‘1104 Publications
REFERENCES
- Moawad FJ. Eosinophilic esophagitis: incidence and prevalence. Gastrointest Endosc Clin N Am. 2018;28(1):15-25.
- Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):281-296.
- Company press release: https://revolobio.com/2023/07/18/revolo-biotherapeutics-announces-additional-data-from-phase-2a-trial-of-1104-in-adults-with-active-eosinophilic-esophagitis/
- Müller S, Maintz L, Bieber T. Treatment of atopic dermatitis: Recently approved drugs and advanced clinical development programs. Allergy. 2024 Jun;79(6):1501-1515. doi: 10.1111/all.16009. Epub 2024 Jan 8. PMID: 38186219.
- Company press release: https://revolobio.com/2024/09/18/revolo-announces-new-preclinical-data-further-validating-atopic-dermatitis-as-a-target-indication-for1104/
- Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS, eds. WAO White Book on Allergy: Update 2013. Executive Summary. Milwaukee, WI: World Allergy Organization; 2013. https://www.worldallergy.org/UserFiles/file/ExecSummary-2013-v6-hires.pdf. Accessed May 4, 2021.
- Internal Market Research
- Piper Atopic Dermatitis Industry Overview Report
- Backman et al. 2018. Severe asthma among adults: Prevalence and clinical characteristics” Eur Resp J.
- Bulow et al. 2014. Low Asthma Control Among Danish Adults. J Allergy and Clinical Immunology.
- CDC Asthma Reports. Atopic Disease in America study (Asthma and Allergy Foundation of America.
- Internal Market Research