Our Science
Addressing the Immune System Response Upstream
Our Revolutionary Approach
People with allergic diseases often have overactive, dysfunctional immune systems characterized by elevated numbers of inflammatory immune cells, which leads to tissue destruction and allergy symptoms.
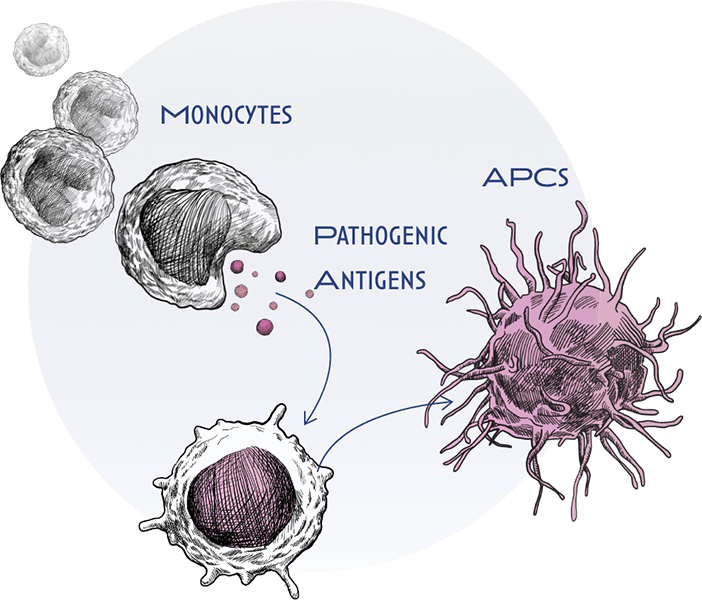
In allergic disease, the immune system “overreacts” to antigens. As part of that recognition, a type of immune cell known as a monocyte, interacts with and engulfs these antigens and then matures into specialized immune cells called antigen presenting cells (APCs), such as macrophages.
APCs interact with other types of immune cells, called naïve T cells and B cells. They then deliver activating signals, which instruct these naïve cells to become inflammatory T or B effector cells. Upon activation, T and B effector cells multiply and release potent inflammatory mediators that drive the chronic inflammation and tissue destruction. This occurs because our immune system ‘loses tolerance’ to antigens that would not usually induce these strong inflammatory responses.

Most allergic disease treatments work downstream on only a limited number of pathways, to address the inflammatory response once the immune response has been activated.
This approach limits long-term treatment efficacy and clinical symptom improvement, with low rates of disease remission. In addition, it can lead to serious infections and life-threatening side effects linked to immunosuppression. For certain diseases like eosinophilic esophagitis (EoE), current treatment options like JAK inhibitors have black box warnings, highlighting the unmet need for safe and efficacious options for patients. The one-dimensional approach of current therapies (with no or limited impact on the regulatory response) relegates their use to chronic administration.
‘1104 – Our Allergic Disease Platform
Revolo’s lead drug product candidate, ‘1104, targets the immune cascade upstream, shifting the immune system from a pro-inflammatory state to its normal regulated, homeostatic state.
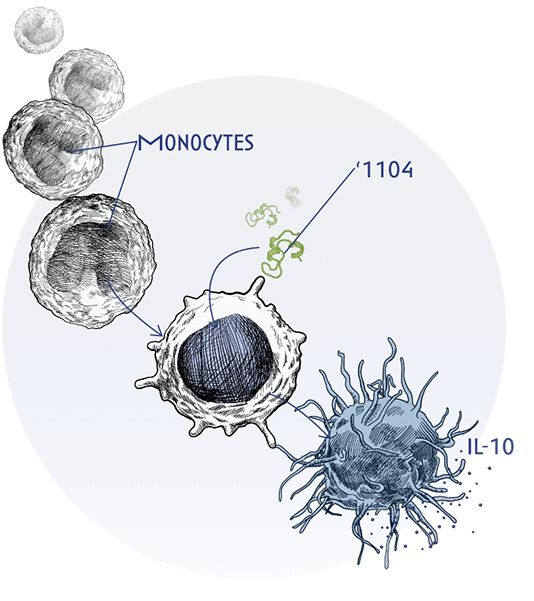
This is achieved through ‘1104’s ability to interact with monocytes and APCs, such as macrophages, instructing downstream pathways and cells both on the effector and regulatory arms of the immune response to help restore immune homeostasis.
‘1104 uniquely binds to a novel receptor in macrophages, reducing IL-4 and IL-13 signaling pathways by activating SHP-1, a molecule that helps turn off these inflammatory signals. In addition, through the same receptor, ‘1104 is able to increase the release of IL-10, a potent anti-inflammatory mediator.
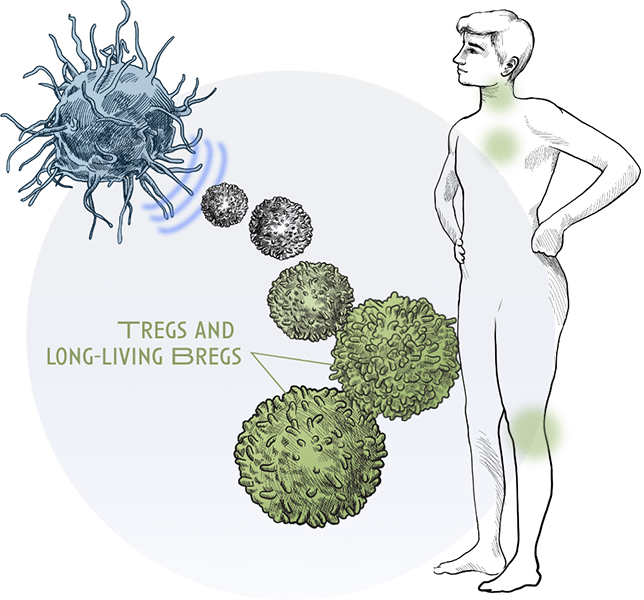
Additionally, ‘1104 rapidly increases the number of activated regulatory T and B cells (T and B regs) instead of proinflammatory T and B effector cells. Regulatory T and B cells are critical in preventing the immune system from entering overdrive. They modulate the immune system, maintain tolerance to self-antigens and prevent allergic disease.
‘1104 was derived from a natural tolerogenic protein, Chaperonin 60.1, involved in regulating the immune system.
‘1104’s Demonstrated potential:
Upstream approach: ‘1104’s upstream effect on the effector and regulatory arms of the immune system has been demonstrated across multiple clinical trials.
Clinical potential and favorable safety profile: Its clinical potential has already been demonstrated in Phase 2 trials in eosinophilic esophagitis (EoE). ‘1104 showed rapid translation to persistent clinical response and favorable safety profiles, with no immune suppression.
Administration route optionality: Preclinical studies comparing ‘1104 intravenous vs subcutaneous (SQ) and oral sublingual (SL) administration has substantiated the viability of all three routes of administration. Subcutaneous delivery of ‘1104 can be highly differentiated while sublingual creates an unprecedented dosing optionality for ‘1104 as compared to current therapies. Both SQ and SL dosage forms are being considered for clinical investigation.
Less frequent treatment regimens: Clinical studies to date have shown that T and B regs upregulation with ‘1104 is maintained for multiple weeks, which supports less frequent treatment regimens, critical for the treatment of chronic diseases like allergies.
Indication versatility: ‘1104’s mechanism of action creates vast optionality across multiple indications given that it works independently of the specific allergen.
'1805 – Our Autoimmune Disease Platform
‘1805 is our second drug candidate in development for the treatment of autoimmune diseases. In these diseases, the body mistakenly recognizes its own tissues (self-antigens) as foreign and attacks them. Similar to allergic diseases, this results in a broad inflammatory response, leading to the symptoms characteristic of these conditions.
‘1805 is a modified analogue of the endogenous immune-regulatory Binding immunoglobulin Protein (BiP), a key player in immune function. Like ‘1104, ‘1805 acts by regulating the immune system upstream through interacting with pro-inflammatory monocytes.
‘1805’s upstream mechanism of action has been demonstrated in clinical and preclinical clinical studies in rheumatoid arthritis.